Treatment for Androgenetic Alopecia (AGA) (Self-pay)
Androgenetic alopecia (AGA) is a form of progressive hair loss that begins after puberty. It typically starts to become noticeable in the late twenties to thirties and gradually advances, often reaching its full extent after age forty. Our clinic provides AGA treatment on a self-pay basis (not covered by insurance).
Prevalence and Progression Patterns of Androgenetic Alopecia (AGA)
Prevalence increases with age: roughly 10% of people in their 20s, 20% in their 30s, 30% in their 40s, and more than 40% over age 50. The speed and degree of progression vary from person to person and are influenced by factors such as genetics and sensitivity to androgens (male hormones).
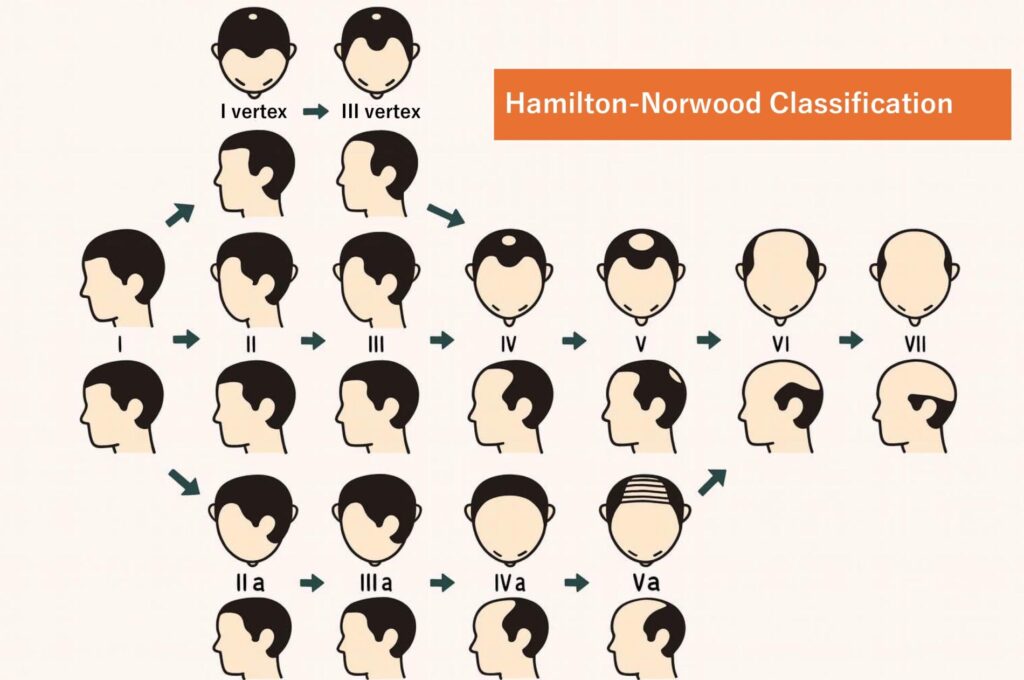
The most commonly used system for assessing the pattern and severity of AGA is the Hamilton–Norwood classification. Based on this scale, AGA progression can be broadly divided into three main patterns. Among Asian populations, including Japanese individuals, the U-shaped pattern is reported to be the most common, followed by the O-shaped pattern.
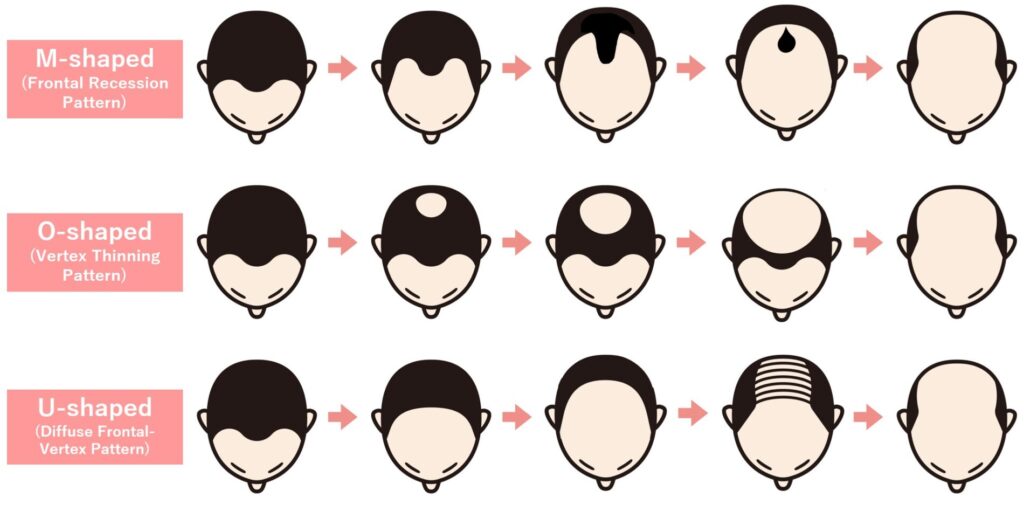
M-shaped pattern (Frontal Recession Pattern)
This pattern starts with recession at both temples, forming an M-shaped hairline that slowly moves backward toward the crown. Because the change is visible in the mirror, most people notice it relatively early.
O-shaped pattern (Vertex Thinning Pattern)
Thinning begins at the crown in a circular area and gradually widens. Many people don't recognize it till late because, unlike the M-shape, the crown is hard to see without intentionally being checked. Some become aware only after someone else points it out. As a result, diagnosis and treatment often tend to occur at a more advanced stage, which can limit the effectiveness of treatments.
Diffuse Frontal–Vertex Pattern (U-shaped)
In this pattern, the entire frontal area—not just the temples—recedes gradually toward the crown. Thinning at the crown, like that seen in the O-shaped pattern, may also develop, and both can progress at the same time. Because it affects a wider area than the M- or O-shaped types, it often becomes more significant.
Pathogenesis of Androgenetic Alopecia (AGA)
Human hair grows from hair follicles. A single follicle typically undergoes about 10 to 30 hair cycles before eventually ceasing to function. This cycle includes a growth phase (anagen) that normally lasts two to six years. In AGA, the core problem is a shortening of the growth (anagen) phase, which increases the proportion of follicles stuck in the resting (telogen) phase.
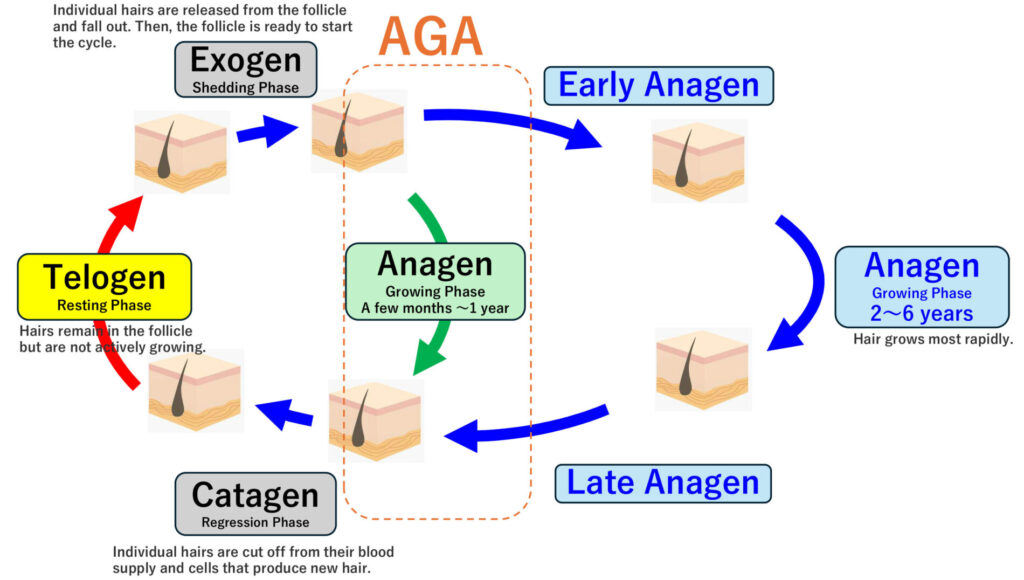
As the growth phase shortens, the hair cycle accelerates, leading to more frequent shedding. In addition, hairs that should grow long and thick stop growing prematurely and remain short and fine—this process is called miniaturization. Eventually, the hair may no longer protrude from the scalp at all.
Androgen secretion increases during the teenage years and peaks in the twenties. While androgens help build bone and muscle and promote thicker body hair (such as beard and chest hair), they paradoxically trigger miniaturization in androgen-sensitive follicles located at the hairline and crown.
Within these androgen–sensitive follicles, dermal papilla cells express androgen receptors. Testosterone (the primary androgen), once transported to the dermal papilla cells of the beard, forehead, and crown, is converted into the more potent dihydrotestosterone (DHT) by Type II 5α-reductase. DHT then binds to androgen receptors. When DHT binds to these receptors in follicles on the forehead and crown, it suppresses the proliferation of matrix cells and shortens the growth phase. As noted earlier, genetic factors—including polymorphisms in the androgen receptor gene on the X chromosome—contribute to individual differences in how AGA progresses.
Treatment for Androgenetic Alopecia (AGA)
Based on the mechanisms described above, current AGA treatments focus on two main approaches:
- Normalizing the hair cycle
- Thickening (reversing miniaturization of) affected hairs
Normalizing the hair cycle
The goal of normalizing the hair cycle is to reduce the production of DHT, which suppresses matrix cell activity and shortens the anagen phase. Medications that inhibit type II 5α-reductase, the enzyme that converts testosterone to DHT, are used to lower DHT levels and protect dermal papilla cells from its effects. The primary medications are finasteride (a type II 5α-reductase inhibitor) and dutasteride (a type I and type II 5α-reductase inhibitor).
Clinical trials of finasteride have shown:
- At 1 year: 58% of patients had at least slight improvement on crown photographs
- At 2 years: 68% showed improvement
- At 3 years: 78% showed improvement (Reference 1)
Other studies report improvement in 99.4% of patients after 5 years of continuous use (Reference 2).
Benefits tend to be greater in patients under age 40 and those with milder symptoms. In a comparative study, both finasteride and dutasteride were effective at 6 months, with dutasteride showing slightly higher efficacy (Reference 3).
These medications do not produce immediate results. Treatment must be continued for at least 6 months before assessing effectiveness. If discontinued, any gains will gradually be lost.
Thickening (Reversing Miniaturization of) Affected Hair
To thicken miniaturized hairs, treatment aims to improve blood flow to the matrix cells, enhancing oxygen and nutrient delivery and promoting hair growth. The medication used for this purpose is Minoxidil. Minoxidil is a vasodilator originally developed to treat high blood pressure. For AGA, it is used as a topical solution. It is also thought to act directly on the hair follicle to prolong the growth phase. Clinical studies have shown increases in both the number of non-miniaturized hairs and the total number of hairs per square centimeter after 6 to 12 months of use.
Side Effects of Finasteride and Dutasteride
Relatively common side effects include decreased libido, erectile dysfunction, and ejaculatory disorders. In clinical trials of dutasteride, these occurred in 8.3%, 11.7%, and 5.0% of patients, respectively, during the first year of use.
These medications also reduce serum PSA (prostate-specific antigen) levels by approximately 50%. Therefore, for patients taking them for longer than 6 months, PSA results obtained for prostate cancer screening should be interpreted by doubling the measured value. PSA levels generally return to baseline approximately six months after discontinuing the medication.
Both dutasteride and finasteride may cause a reduction in sperm count, semen volume, and sperm motility (with dutasteride showing about a 20% decrease in these parameters). As a result, male infertility can occur, although in most cases sperm parameters return to baseline about six months after stopping the medication. For men who are actively trying to conceive, starting these medications requires careful consideration.
Dutasteride and finasteride were originally developed as treatments for benign prostatic hyperplasia (BPH). They reduce prostate volume and help improve urinary symptoms. Some clinical studies suggest a potential reduction in the overall risk of developing prostate cancer; however, other data indicate a possible increased risk of higher-grade prostate cancer. There have also been rare reports of male breast cancer in patients taking these medications, although a causal relationship has not been established.
Other potential adverse effects—though uncommon—include elevated liver enzymes, depressed mood, altered taste, dizziness, urticaria, and angioedema.
Side Effects of Minoxidil
Local skin reactions such as redness, flaking, folliculitis, contact dermatitis, and unwanted facial hair growth occur in roughly 6% of users.
AGA treatment at Park East Clinic
This combined approach—enhancing hair growth by prolonging the follicle’s growth phase with oral Finasteride or Dutasteride, and improving blood supply and prolonging growth phase with topical Minoxidil—is recommended by the Japanese Dermatological Association as a standard treatment strategy for AGA (Reference 4).
In line with this current recommendations, our approach to AGA treatment includes oral finasteride or dutasteride and the use of topical minoxidil.
The initial visit and any appointment that requires blood work must be done in person. All other follow-up visits can be conducted online if preferred.
We perform blood testing one month after starting oral medication to confirm safety. However, if you have had the required labs drawn at another medical facility, and the results include all necessary parameters, you may share those results with us and skip the blood draw at our clinic.
Treatment Costs for Androgenetic Alopecia (AGA)
- Initial consultation: ¥3,000 (including tax) for both in-person and online
- Follow-up consultation: ¥1,000 (including tax) for both in-person and online
- Finasteride (brand: "PROPECIA") 1mg – ¥10,800/box (28 capsules, tax included)
- Finasteride (generic: Sawai) 1mg – ¥5,400/box (28 capsules, tax included)
- Dutasteride (brand: "ZAGALLO") 0.5mg – ¥12,000/box (30 capsules, tax included)
- Dutasteride (generic: Me Pharma) 0.5mg – ¥6,300/box (30 capsules, tax included)
- Minoxidil (5% topical solution) – TBA
- Laboratory test fee: ¥4,800 (including tax)
- Conducted one month after starting the medication to assess safety.
- May be performed subsequently if deemed necessary for safety evaluation at needed frequencies.
- Shipping fee (for online consultations): ¥2,000 (including tax)
- Communication fee (for online consultations): ¥1,000 (including tax)
References
- 川島 眞,溝口将之,五十嵐敦之ほか:男性型脱毛症(AGA)に対するフィナステリドの長期投与(3 年間)試験成績 多施設共同オープン試験,臨皮,2006; 60: 521―530
- Yoshitake T, Takeda A, Ohki K, et al: Five-year efficacy of finasteride in 801 Japanese men with androgenetic alopecia, J Dermatol, 2015; 42: 735―738
- J Am Acad Dermatol. 2014 Mar;70(3):489-498
- The Japanese Dermatological Association Guidelines for the Management of Androgenetic Alopecia and Female Pattern Hair Loss 2017 Edition

