Obesity treatment (not covered by NHI)
Our Approach to Obesity Treatment (Mounjaro)
- Lose weight safely and at your own pace, under the care of a board-certified internal medicine physician.
Our clinic offers obesity treatment with Mounjaro (not covered by NHI) for individuals who have struggled to achieve weight loss through diet and exercise or who have limitations on physical activity due to pain in their limbs or trunk, or conditions affecting the heart or lungs.
As a board-certified internist, our director provides you with a full range of care, including a safety assessment before starting treatment, ongoing monitoring of safety and effectiveness after treatment begins, and individualized guidance on diet and exercise. Our goal is to help you move toward your target weight safely and at your own pace.
However, we do not offer treatment for weight loss solely for cosmetic purposes. Specifically, treatment is generally not provided to individuals whose BMI* is already below 18.5—the lower limit of the “normal weight” criteria—or for those who aim to reduce their BMI to below 18.5.
| ※BMI | BMI is a simple screening tool to classify weight status (underweight, normal weight, overweight, or obese) widely used around the world. |
| BMI formula | BMI = weight(kg) ÷ {height(m) × height(m)} |
| BMI | Category |
| ~ 18.4 | Underweight |
| 18.5~24.9 | Healthy weight |
| 25.0~29.9 | Overweight |
| 30.0~34.9 | Obesity(Class 1) |
| 35.0~39.9 | Obesity(Class 2) |
| 40.0 ~ | Obesity(Class 3) |
What is tirzepatide / Mounjaro / Zepbound ?
Tirzepatide (generic name) was originally developed as a treatment for type 2 diabetes (administered via a weekly subcutaneous injection). It was approved by the U.S. Food and Drug Administration (FDA) on May 13, 2022, and in Japan on September 26, 2022, and has been widely used by patients with diabetes. Due to its appetite-suppressing and weight-loss effects, tirzepatice has also gained attention as an obesity treatment. In the U.S., it was approved as an obesity treatment under the brand name Zepbound by the FDA on November 8, 2023, and in Japan on December 27, 2024, for individuals with a BMI of 30 or higher, or a BMI of 27 or higher with at least one weight-related condition such as hypertension, dyslipidemia, type 2 diabetes, obstructive sleep apnea, or cardiovascular disease.
Tirzepatide works by binding to and activating the receptors of two hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), improving blood sugar control by stimulating insulin secretion in response to blood sugar levels. It also suppresses appetite and caloric intake, leading to weight loss. This effect is believed to be primarily due to its influence on brain regions that control hunger and satiety signals, reducing the feeling of hunger and slowing the movement of food from the stomach to the intestines, thereby prolonging the feeling of fullness. It is also believed that tirzepatide achieves weight loss via a slight but significant increase in energy expenditure (fat burning) (Mol Metab 2018;18:3–14).
In a clinical trial (SURMOUNT-1) involving obesity patients (average weight 104.8 kg) from nine countries including the U.S., Japan, and China, tirzepatide treatment (5 mg, 10 mg, 15 mg) resulted in a weight reduction of 15.0%, 19.5%, and 20.9%, respectively, after 72 weeks. The graph on the right shows that the effect is seen immediately after starting the treatment. Additionally, the majority of the weight loss (three-quarters) came from the loss of fat.
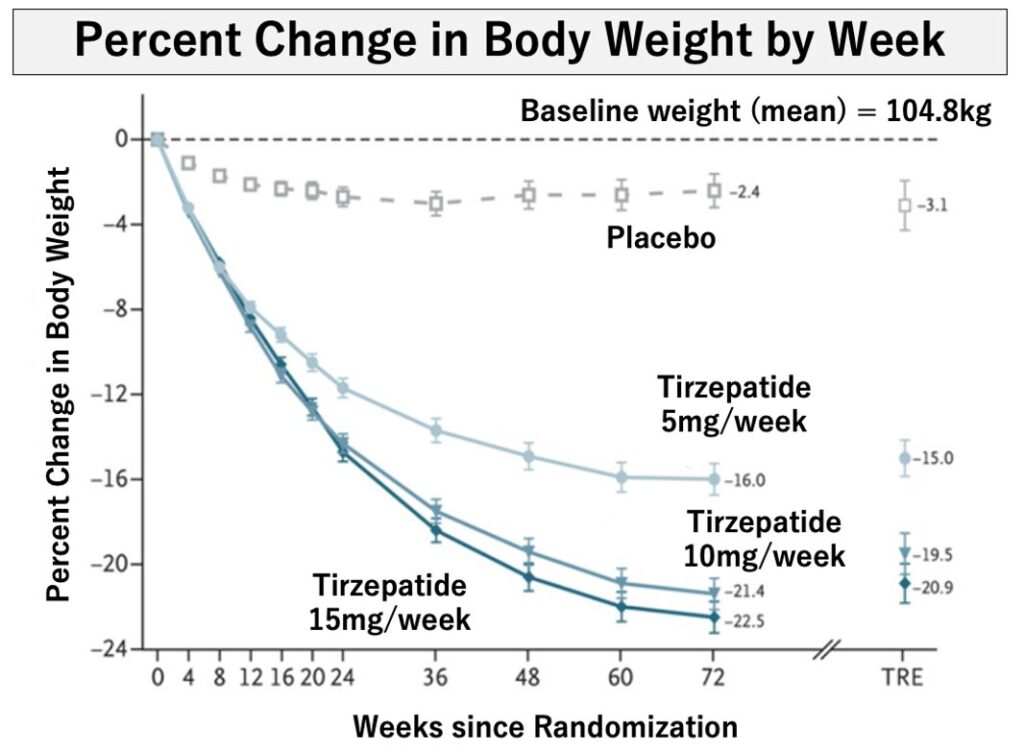
N Engl J Med 2022;387:205-216
A separate study, SURMOUNT-J, focused specifically on Japanese patients (average weight 92.0 kg (203 lbs)). Tirzepatide treatment (10 mg and 15 mg) resulted in a weight reduction of 16.1% and 21.1%, respectively, after 72 weeks.
The most common side effects of tirzepatide include gastrointestinal symptoms such as nausea, vomiting, diarrhea, and constipation, experienced by about 30% of patients, especially in the early stages of treatment. Additionally, as with other medications acting on GLP-1 receptors, there is a reported increase (1.3~1.5 times) in the risk of acute biliary diseases (cholecystitis, cholangitis). As tirzepatide is the first medication to act on both GLP-1 and GIP receptors, its long-term safety data are still being accumulated.
Mounjaro Treatment at Our Clinic is NOT covered by NHI
As mentioned above, tirzepatide has already been approved in the United States as an anti-obesity medication under the brand name Zepbound. Following this, Japan also approved Zepbound as a treatment for obesity on December 27, 2024. However, similar to semaglutide, which has already been approved in Japan both as a diabetes medication (Ozempic) and an anti-obesity drug (Wegovy), the prescription of Zepbound under NHI is limited to certain medical institutions that meet the following strict facility criteria. Realistically, NIH-covered prescriptions are available only at university hospitals and large general hospitals.
【Tirzepatide】 https://www.hospital.or.jp/site/news/file/1742282783.pdf
【Semaglutide】 https://kouseikyoku.mhlw.go.jp/kyushu/000296496.pdf
Under the circumstance, with the strong passion to assist individuals who have struggled to achieve weight loss through diet and exercise or who have limitations on physical activity due to pain in their limbs or trunk, or conditions affecting the heart or lungs, our clinic currently offers tirzepatide (Mounjaro is the only available option for purchase at clinics) as a self-pay (non-insurance) treatment option in which all costs (100%) are borne by the patient.
Obesity Treatment with Mounjaro at Our Clinic
Our goal is to assist individuals who have struggled to achieve weight loss through diet and exercise or who have limitations on physical activity due to pain in their limbs or trunk, or conditions affecting the heart or lungs. We do not offer treatment for weight loss solely for cosmetic purposes. Specifically, treatment is generally not provided to individuals whose BMI* is already below 18.5—the lower limit of the “normal weight” criteria—or for those who aim to reduce their BMI to below 18.5.
If/when a patient’s BMI drops below 18.5 during treatment, prescriptions will be limited to what is necessary to maintain a normal BMI (18.5 or above). To avoid health issues caused by the treatment, blood tests and examinations are mandatory before starting treatment (only if deemed necessary for safety assessment) and regularly thereafter (after the first 4 treatments, then every 12 treatments). Results from tests conducted at other medical institutions within a certain period can be shared as a substitute.
Initial Visit Includes:
- Physical measurements (height, weight, waist circumference)
- Medical interview
- Physical examination
- (If deemed necessary for safety assessment) Blood tests (CBC, HbA1c, blood sugar, liver function, kidney function, pancreatic enzymes)
The initial prescription is limited to 4 pens for safety reasons, and up to 12 pens for subsequent prescriptions if clinically appropriate.
After the first 4 treatments, and every 12 treatments thereafter if treatment continues, the same blood tests as before starting will be conducted. Results from recent tests at other medical institutions can substitute for the tests at our clinic.
The initial consultation is generally conducted in person, but follow-up consultations can be conducted online if no blood tests are required.
Fees:
- Initial consultation: 3,000 yen (including tax) for both in-person and online
- Follow-up consultation: 1,000 yen (including tax) for both in-person and online
- Test fees (blood tests, height, and weight measurement): 4,800 yen (including tax)
- Mounjaro 2.5 mg pen (each): 4,800 yen (including tax)
- Mounjaro 5 mg pen (each): 6,800 yen (including tax)
- Mounjaro 7.5 mg pen (each): 9,200 yen (including tax)
- Mounjaro 10 mg pen (each): 11,500 yen (including tax)
- Mounjaro 12.5 mg pen (each): 15,000 yen (including tax)
- Mounjaro 15 mg pen (each): 18,000 yen (including tax)
- Shipping fee (for online consultations): 2,000 yen (including tax)
- Communication fee (for online consultations): 1,000 yen (including tax)
Sample treatment flow
If 2.5 mg is sufficient for weight loss:
First Visit (in-person, no initial safety blood test):
Initial consultation fee + 2 Mounjaro 2.5 mg pens
Total: 12,600 yen
First Visit (in-person, with initial safety blood test):
Initial consultation fee + test fee + 2 Mounjaro 2.5 mg pens
Total: 17,400 yen
Second Visit (2 weeks later, online):
Follow-up consultation fee + 2 Mounjaro 2.5 mg pens + shipping fee + communication fee
Total: 13,600 yen
Third Visit (2 weeks later, in-person):
Follow-up consultation fee + test fee + 4 Mounjaro 2.5 mg pens
Total: 25,000 yen
Fourth Visit (4 weeks later, online):
Follow-up consultation fee + 4 Mounjaro 2.5 mg pens + shipping fee + communication fee
Total: 23,200 yen
Fifth Visit (4 weeks later, online):
Follow-up consultation fee + 4 Mounjaro 2.5 mg pens + shipping fee + communication fee
Total: 23,200 yen
Sixth Visit (4 weeks later, in-person):
Follow-up consultation fee + test fee + 4 Mounjaro 2.5 mg pens
Total: 25,000 yen
If 2.5 mg is insufficient, and increased to 5 mg:
First Visit (in-person, no initial safety blood test):
Initial consultation fee + 2 Mounjaro 2.5 mg pens
Total: 12,600 yen
First Visit (in-person, with initial safety blood test):
Initial consultation fee + test fee + 2 Mounjaro 2.5 mg pens
Total: 17,400 yen
Second Visit (2 weeks later, online):
Follow-up consultation fee + 2 Mounjaro 5 mg pens + shipping fee + communication fee
Total: 17,600 yen
Third Visit (2 weeks later, in-person):
Follow-up consultation fee + test fee + 4 Mounjaro 5 mg pens
Total: 33,000 yen
Fourth Visit (4 weeks later, online):
Follow-up consultation fee + 4 Mounjaro 5 mg pens + shipping fee + communication fee
Total: 31,200 yen
Fifth Visit (4 weeks later, online):
Follow-up consultation fee + 4 Mounjaro 5 mg pens + shipping fee + communication fee
Total: 31,200 yen
Sixth Visit (4 weeks later, in-person):
Follow-up consultation fee + test fee + 4 Mounjaro 5 mg pens
Total: 33,000 yen

